Little Silver Pediatrics and Family Medicine • 200 White Road • Suite 212 • Little Silver, NJ 07739 732-741-5600

The Food and Drug Administration authorized the Moderna and Pfizer COVID-19 vaccines for the youngest children on Friday, June 17, following a meeting of its advisory committee, which also endorsed the vaccines. With the CDC’s formal endorsement, children as young as 6 months will be able to begin receiving COVID-19 vaccines.
Some of the local pharmacies have already started receiving their supplies. Please check with your local pharmacy about availability for your child.
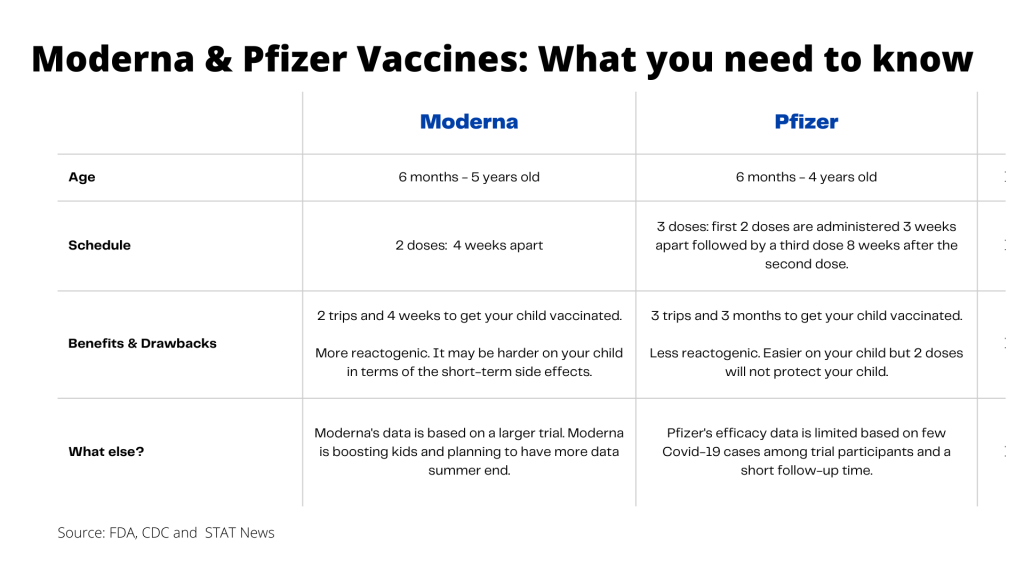
There are two vaccines – Pfizer and Moderna. Which do you choose?
Please review the following information to decide which vaccine is best for your child. In this age group, the Pfizer-BioNTech and the Moderna vaccines probably differ more than they do in any other age group on the vaccination spectrum.
The Pfizer-BioNTech vaccine is authorized for children 6 months to 4 years of age. The Moderna vaccine is authorized for children 6 months to 5 years of age.
Dosing differences
The two manufacturers took very different approaches. There are benefits and potential drawbacks for each of them as a result. It remains to be seen which choice was the smartest.
Pfizer focused on tolerability by choosing a small dose, figuring that if a vaccine caused too many side effects that made kids miserable parents might not bring their children back for additional doses. The company’s vaccine contains just 3 micrograms of antigen. That’s one-tenth of the amount of vaccine in its adult dose.
But two doses of the Pfizer vaccine, given 21 days apart, didn’t trigger a strong enough immune response in young children, and the company had to add a third dose to its regimen. The third dose is critical. Without it, children are likely not protected. After the second dose, there appears to be “very little or no effectiveness,” said panel member Amanda Cohn, a vaccines expert from the Centers for Disease Control and Prevention, who noted the need for a third dose means “these kids would not be protected at all for an additional eight weeks.’’
Moderna, on the other hand, developed a two-dose vaccine that contains 25 micrograms in each shot. That’s one-quarter of the amount an adult dose contains. The shots are given four weeks apart, after which a child would be termed fully vaccinated or up to date on their Covid vaccines.
The bigger dose paid off. Moderna cleared the FDA’s bar for approval with just two doses.
The FDA requirement was that the manufacturers show their vaccines induced in young children with similar levels of antibodies as they did in young adults.
The Benefits and Drawbacks
The benefits of the Moderna vaccine: Only two trips to the Health Department Clinic or local pharmacy completes the primary series. One fewer dose to have to cajole a toddler into getting the shot. Quicker protection.
The benefits of the Pfizer vaccine: Milder reactions.
The drawback of the Moderna vaccine: These bigger doses of vaccine sound like they may be harder on kids, in terms of transient side effects, than the Pfizer vaccine. (More on side effects below.)
The drawback of the Pfizer vaccine: It will take nearly three months to get a child fully vaccinated. Getting this vaccine requires three medical visits, and three vaccinations in an age group that typically hates getting needles. Failing to get the third shot means a child probably wouldn’t be protected.
Vaccine efficacy
The vaccines were authorized for this age group based on the comparisons of the immune responses measured by antibody levels. For the two-dose Moderna vaccine, it’s also possible to measure the efficacy of the vaccine by comparing the number of times volunteers in the 6,400-person clinical trial started feeling sick and tested positive for Covid.
Among kids who were six to 23 months old, the Moderna vaccine was 50.6% effective. That means there were about 7 cases of Covid for every hundred children that received placebo, compared to about 3.5 for every 100 who got the vaccine. In kids aged 2 to 5, the vaccine efficacy was 36.8%. That determination is based on 180 Covid cases.
But for the three-dose Pfizer vaccine, there have only been 10 Covid cases spread across the vaccine and placebo groups, meaning the FDA and CDC don’t feel comfortable estimating the vaccine’s efficacy.
This was a result of the fact that the Pfizer shot was not effective enough as a two-dose vaccine to warrant authorization. The company, consulting with the FDA, decided to test a third dose, but there has simply not been enough time for cases of Covid to occur. FDA officials say they are confident the three-dose regimen protects as well as two doses in other age groups.
Additional resources:
Covid-19 Vaccines for Children & Teens | CDC
